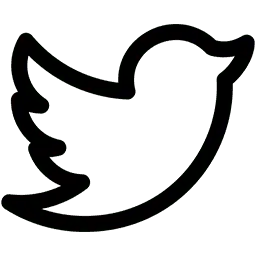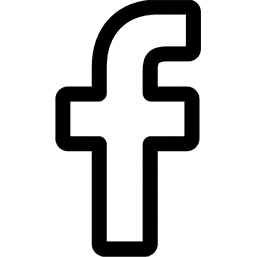Kentucky Bioprocessing in Owensboro, Ky, has the ability to be a supplier of ZMapp, an experimental serum aimed at boosting the immune system’s efforts to fight off Ebola. (Jenny Sevcik/ Messenger-Inquirer via Associated Press)
Tomorrow the World Health Organization will convene a phone meeting of experts from around the world to discuss the ethical issues of providing experimental treatment to people suffering from Ebola, a highly fatal disease that is spreading in four West African countries. The WHO phone meeting follows the administration of ZMapp — an experimental serum to fight Ebola — to two American missionaries infected with Ebola in Liberia. Both missionaries are now being treated in the United States, and their health appears to be improving.
Last week my colleague Laura Seay weighed in on the ethics of providing experimental Ebola treatment. Laura and I are good friends and often agree on a number of issues – but our opinions diverged on this issue. In her well-written and well-argued post, she highlights a particular problem rooted in the history of medical research:
In the case of medical trials, ethical researchers are also aware of the long and terrible history of research — often on poor black people who were not asked to give informed consent — that has caused significant harm to study participants. From the Tuskegee syphilis experiment to Pfizer’s disastrous 1996 meningitis vaccine trial in Nigeria, unethical studies that victimize vulnerable persons of African origin abound. Nobody involved in discussions about ZMapp wants to repeat this situation, or to put West African Ebola patients who may or may not be able to meaningfully consent at risk for consequences no one can anticipate.
It is on this issue of experimenting on vulnerable populations that I completely agree with Laura. The terrible history of the use of vulnerable populations by research companies and governments in ways that were not meant to benefit those populations and in fact put those populations through undue harm should keep us alert and vigilant against any action that might bear resemblance.
The point of my departure, however, is seeing the provision of experimental Ebola treatment to West Africans as a question of research ethics rather than a question of treatment ethics. As sociologist Amy Kaler noted in a comment on Laura’s post:
… this piece conflates research ethics with treatment ethics. The situation in west Africa is not a research situation, it’s more analogous to an emergency ward or a disaster zone. The author is quite right that research ethics require participants to be capable of giving fully informed consent, but emergency medical procedures are often carried out on people who are not capable of informed consent. If the man in line ahead of me at Starbucks suddenly fell over and stopped breathing, his ability to consent to medical treatment would not be at issue in making the decision whether to start CPR.
When I say I think West Africans should have access to experimental treatment, I’m not saying that the company manufacturing ZMapp (or the other companies with experimental Ebola treatments) should be allowed to run clinical trials that disregard ethical principles on unhealthy West Africans to learn whether their treatment is effective and safe. Rather, I’m saying that West Africans suffering from Ebola should have the same access to treatment and care that Americans have. (And if the former also happens under conditions that pose no additional risk or harm to patients, that’s fine by me.)

Peter Piot, director of the London School of Hygiene and Tropical Medicine, has deemed the Ebola outbreak exceptional and called for a “limited deployment” of the best experimental treatments. (Leon Neal/Agence France-Presse via Getty Images)
Leading global health scholars have weighed in. Jeremy Farrar (Wellcome Trust), David Heymann (Chatham House Center on Global Health Security) and Peter Piot (London School of Hygiene and Tropical Medicine) published an op-ed in the Wall Street Journal (ungated) in which they deemed the Ebola outbreak as exceptional and call for a “limited deployment” of the best experimental treatments and offer some guidance on how to provide those treatments while also minimizing harm and working under the constraints of scarcity.
The ethical dilemma that seems more pertinent to me in the current response to Ebola is in the governance of global health inequalities. Jennifer Prah Ruger, faculty at Yale in Medical Ethics and Health Policy, summarized global health inequalities with a few key statistics:
…a child born today in Afghanistan is 75 times as likely to die by age 5 years as a child born in Singapore. A girl born in Sierra Leone can expect to live 50 fewer years, on average, than her Japanese counterpart. The number of African children at risk of dying is 35% higher today than it was 10 years ago. Although the average global life expectancy has increased by 20 years over the past five decades, the poorest countries have been left behind.
The differential treatment of patients in the current Ebola outbreak is but a magnification of the inequality of health provision: White Americans get experimental treatment. Black Africans don’t. In her paper, Ruger tries to identify a moral framework for solving problems of global health justice – and one principle seems rather straightforward: an equal respect for all human life. If there is an equal respect for all human life, why do ethical issues stand as barriers for one population of people, but not for another?
The question of treatment ethics and the inequality in health-care provision that I raise here does not address other issues raised in Laura’s post and others on the ethics of making ZMapp available on a wider scale to West Africans suffering from Ebola (e.g., informed consent, exposing patients to potentially severe side effects yet unknown because of lack of human testing, etc.). I write this to point out that these issues did not stop Kent Brantly or Nancy Writebol from receiving the ZMapp treatment (there are also reports that a Spanish priest infected with Ebola in Liberia and evacuated to Spain will receive ZMapp treatment). The decision on whether Brantly and Writebol could get ZMapp didn’t require the WHO to convene a panel of ethicists. Why must West Africans wait?
If we use an ethical argument to prohibit use of a medical treatment, it should be equally administered. When exceptions are made for people from privileged populations, the ethical problem is less about using an untested treatment and more about equal access to treatment.
****
This is now the fourth Monkey Cage post about the politics of responding to the Ebola outbreak in West Africa. See our earlier posts: